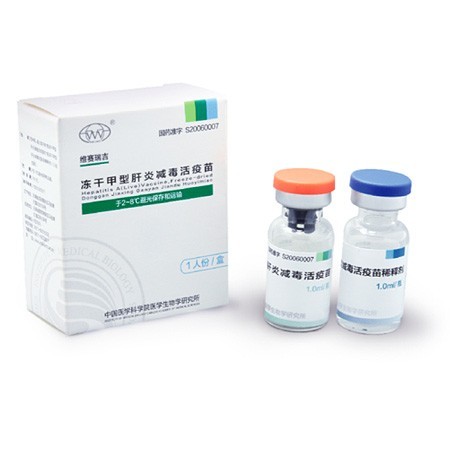
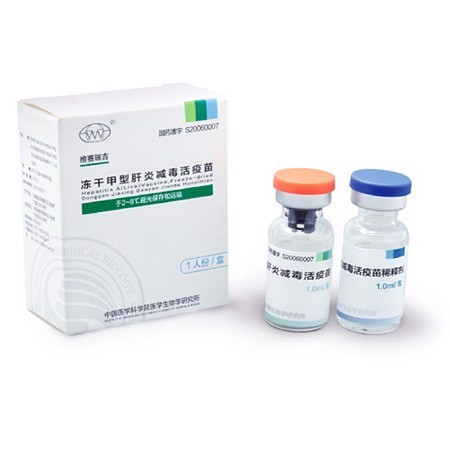
Hepatitis A (Live) Vaccine, Freeze-Dried
Product Advantages:
The first viral vaccine which holds the independent intellectual property in China.
The National Torch Program’, ‘The National Major New Products’.
Over 200 million doses have been provided.
The market share in China ranks among the top.
- <p style="white-space: normal"><strong>The Representative Research and Production Base for Series of Hepatitis A Vaccines in China</strong></p><p style="white-space: normal">As early as in 1974, IMBCAMS independently established a human diploid cell line, KMB17. Its establishment provides a base for the subsequent development of new vaccines from human diploid cells.</p><p style="white-space: normal">In 1992, the live-attenuated Hepatitis A vaccine (liquid) from IMBCAMS was firstly permitted to market.</p><p style="white-space: normal">The inactivated Hepatitis A vaccine, WEISAIRUIAN®, and the freeze-dried live-attenuated Hepatitis A vaccine, WEISAIRUIJI®, were respectively put into Chinese market in 2003 and 2006.</p><p style="white-space: normal"><strong>A Manufacturer Who Remarkably Contributes to Prevent Hepatitis A in China</strong></p><p style="white-space: normal">The Hepatitis A vaccine has been included into the Expanded Program on Immunization (EPI) in 2007.</p><p style="white-space: normal">For more than 20 years, IMBCAMS provided Chinese market about 200 million doses of Hepatitis A vaccines in total.</p>
- <table cellspacing="0"><tbody><tr class="firstRow"><td width="553" valign="center" style="padding: 0 7px; border-color: rgba(0, 0, 0, 1)"><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Main Active Composition:</span></strong></p></td></tr><tr><td width="553" valign="center" style="padding: 0 7px; border-color: initial rgba(0, 0, 0, 1) rgba(0, 0, 0, 1)"><p style="line-height: 21px; vertical-align: baseline"><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Live-attenuated Hepatitis A virus.</span></p></td></tr><tr><td width="553" valign="center" style="padding: 0 7px; border-color: initial rgba(0, 0, 0, 1) rgba(0, 0, 0, 1)"><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Adjuvants:</span></strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px"> Gelatin, Mycose, Glycine, Dextranum.</span></p><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Vaccine Diluent:</span></strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px"> Sterile water for injection.</span></p><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Description:</span></strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px"> Milky white </span><span style="font-family: "Times New Roman"; color: rgba(102, 102, 102, 1); font-size: 14px"><span style="font-family: Arial">crips cake</span></span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">, </span><span style="font-family: "Times New Roman"; color: rgba(102, 102, 102, 1); font-size: 14px"><span style="font-family: Arial">and it turns into a clear liquid after reconstitution</span></span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">.</span></p><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Storage:</span></strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px"> Stored and transported in 2-8</span><span style="font-family: 宋体; color: rgba(102, 102, 102, 1); font-size: 14px">℃</span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">, protected from light.</span></p><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Period of Validity: </span></strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">18 months.</span></p><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Packaging:</span></strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px"> vials.</span></p></td></tr><tr><td width="553" valign="center" style="padding: 0 7px; border-color: initial rgba(0, 0, 0, 1) rgba(0, 0, 0, 1)"><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Indication:</span></strong></p><p style="line-height: 21px; vertical-align: baseline"><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">The vaccine can induce immunity against Hepatitis A virus to prevent hepatitis A. </span></p></td></tr><tr><td width="553" valign="center" style="padding: 0 7px; border-color: initial rgba(0, 0, 0, 1) rgba(0, 0, 0, 1)"><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Usage:</span></strong></p><p style="line-height: 21px; vertical-align: baseline"><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px"> Hepatitis A susceptible</span><span style="font-family: "Times New Roman"; color: rgba(102, 102, 102, 1); font-size: 14px"><span style="font-family: Arial"> </span><span style="font-family: Arial">individuals</span></span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px"> </span><span style="font-family: "Times New Roman"; color: rgba(102, 102, 102, 1); font-size: 14px"><span style="font-family: Arial">above the age of</span></span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px"> 18 month</span><span style="font-family: "Times New Roman"; color: rgba(102, 102, 102, 1); font-size: 14px"><span style="font-family: Arial">s</span></span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">.</span></p></td></tr><tr><td width="553" valign="center" style="padding: 0 7px; border-color: initial rgba(0, 0, 0, 1) rgba(0, 0, 0, 1); word-break: break-all"><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Administration and Dosage:</span></strong></p><p style="line-height: 21px; vertical-align: baseline"><span style="font-family: "Times New Roman"; color: rgba(102, 102, 102, 1); font-size: 14px"><span style="font-family: Arial">Reconstitute the vaccine with sterile water for injection as instructed value. Shake the vial till the content is reconstituted complete before use.</span></span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px"> Subcutaneous injection in the lateral deltoid of the upper arm is recommended for this vaccine. 1.0 ml per person, and only one dose is required. </span></p><p><br></p></td></tr></tbody></table>
Related Products


用户登录
还没有账号?
立即注册