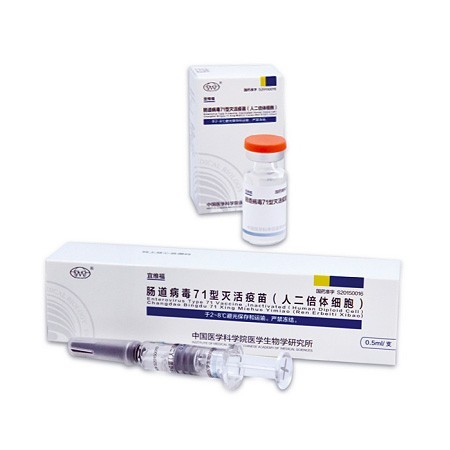
Inactivated Enterovirus Type 71 Vaccine (Human Diploid Cell)
Product Advantages:
The first inactivated EV71 vaccine all over the world
Cultivated in human diploid cell substrate, without any preservative
Suitable to the EV71 susceptible people from 6 months to 5 years old
The efficacy for preventing the EV71-caused HFMD reaches 97.3%
- <p style="white-space: normal"><strong>The First Inactivated Enterovirus Type 71 Vaccine (Human Diploid Cell) All Over the World</strong></p><p style="white-space: normal">After 8 year’s efficient work, EntroVac® , the first vaccine against Hand, Foot and Mouth Disease (HFMD) caused by Enterovirus Type 71 (EV-71) was approved by CFDA in 3rd December, 2015.</p><p style="white-space: normal">Development milestone:</p><p style="white-space: normal">In 2007, Laboratory research was started.</p><p style="white-space: normal">In 2008, a viral strain was isolated in Fuyang, Anhui province, China.</p><p style="white-space: normal">In 2010, the approval to conduct clinical trials was obtained.</p><p style="white-space: normal">In 2013, clinical trials were finished.</p><p style="white-space: normal">In 2015.12.3, approval certificate of EntroVac® was released by CFDA.</p><p style="white-space: normal">In 2016.3.18, EntroVac® was launched to market.</p><p style="white-space: normal">In 2016.3.22, the first dose was immunized.</p><p style="white-space: normal"> </p><p style="white-space: normal"><strong>The Innovative Vaccine from Theory to Practice</strong></p><p style="white-space: normal">Utilizing domestic primate resources, IMBCAMS invented neonatal monkey model to extract pathological features of EV-71 infection and to evaluate protective effect of EntroVac®.</p><p style="white-space: normal">Immune response mechanism induced by EntroVac® was analyzed in clinical trials.</p><p style="white-space: normal">A multi-center phase III clinical trial involving 12000 subjects at 6 to 71 months age sufficiently proved the safety and efficacy of the product. Over two course of epidemic seasons, efficacy of the vaccine was 97.3%. </p><p style="white-space: normal"><strong>The Persistent Research Provides the World Powerful Weapons in Controlling HFMD</strong></p><p style="white-space: normal">EntroVac® created by IMBCAMS is derived from the C4 subtype of EV-71, but the cross-neutralization test suggested that the vaccine could protect against most circulating EV-71 strains in different countries.</p><p style="white-space: normal">At present, scientists in IMBCAMS are still pushing forward the research on other major pathogens of HFMD such as Coxsackie virus A16. </p><p style="white-space: normal">In future, the prospective research of combined vaccines containing different Enteroviruses will provide a stronger strategy in preventing HFMD.</p>
- <table cellspacing="0"><tbody><tr class="firstRow"><td width="553" valign="center" style="padding: 0 7px; border-color: rgba(0, 0, 0, 1)"><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Main Active Composition:</span></strong></p></td></tr><tr><td width="553" valign="center" style="padding: 0 7px; border-color: initial rgba(0, 0, 0, 1) rgba(0, 0, 0, 1)"><p style="line-height: 21px; vertical-align: baseline"><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Inactivated type 71 Enterovirus, the neutralizing antibody titer of EV71 is no less than 3.0EU (containing 100U EV71 antigen)</span></p></td></tr><tr><td width="553" valign="center" style="padding: 0 7px; border-color: initial rgba(0, 0, 0, 1) rgba(0, 0, 0, 1); word-break: break-all"><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Adjuvants:</span></strong><span style="font-family: "Times New Roman"; color: rgba(102, 102, 102, 1); font-size: 14px"><span style="font-family: Arial"> </span></span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Al</span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px"> </span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">(OH)</span><sub><span style="font-family: inherit; color: rgba(102, 102, 102, 1); font-size: 14px; vertical-align: sub">3</span></sub><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">, Glycine</span></p><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Description:</span></strong><span style="font-family: "Times New Roman"; color: rgba(102, 102, 102, 1); font-size: 14px"><span style="font-family: Arial"> </span></span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Slightly milk-white suspension. </span><span style="font-family: "Times New Roman"; color: rgba(102, 102, 102, 1); font-size: 14px"><span style="font-family: Arial">Layering sediment</span></span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px"> may form, but it can disperse by shaking.</span></p><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Storage:</span></strong><span style="font-family: "Times New Roman"; color: rgba(102, 102, 102, 1); font-size: 14px"><span style="font-family: Arial"> </span></span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Stored and transported in 2-8</span><span style="font-family: 微软雅黑; color: rgba(102, 102, 102, 1); font-size: 14px">℃</span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">, protected from light. Avoid freeze.</span></p><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Period of Validity:</span></strong><span style="font-family: "Times New Roman"; color: rgba(102, 102, 102, 1); font-size: 14px"><span style="font-family: Arial"> </span></span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">24 months.</span></p><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Packaging:</span></strong><span style="font-family: "Times New Roman"; color: rgba(102, 102, 102, 1); font-size: 14px"><span style="font-family: Arial"> </span></span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Pre-filled syringes or vials.</span></p></td></tr><tr><td width="553" valign="center" style="padding: 0 7px; border-color: initial rgba(0, 0, 0, 1) rgba(0, 0, 0, 1)"><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Indication:</span></strong></p><p style="line-height: 21px; vertical-align: baseline"><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">After administration, this vaccine can induce immune response to prevent hand, foot, and mouth disease (HFMD) caused by infection of EV71 virus.</span></p></td></tr><tr><td width="553" valign="center" style="padding: 0 7px; border-color: initial rgba(0, 0, 0, 1) rgba(0, 0, 0, 1)"><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Usage:</span></strong></p><p style="line-height: 21px; vertical-align: baseline"><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">The vaccine is suitable for EV71 virus susceptible children aged from 6 months to 5 years old.</span></p></td></tr><tr><td width="553" valign="center" style="padding: 0 7px; border-color: initial rgba(0, 0, 0, 1) rgba(0, 0, 0, 1)"><p style="line-height: 21px; vertical-align: baseline"><strong><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Administration and Dosage:</span></strong></p><p style="line-height: 21px; vertical-align: baseline"><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">Before use, the vaccine should be shaken well. Two doses of vaccines should be used in </span><span style="font-family: "Times New Roman"; color: rgba(102, 102, 102, 1); font-size: 14px"><span style="font-family: Arial">one month interval</span></span><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px">. Recommended intramuscular injection into the deltoid muscle of upper arm and 0.5ml per person per time.</span></p><p><span style="font-family: Arial; color: rgba(102, 102, 102, 1); font-size: 14px"><br></span></p></td></tr></tbody></table><p><br></p>
Related Products


用户登录
还没有账号?
立即注册